NeuDirection Limited has achieved a significant scientific breakthrough with its lead asset, Neu-001, in the field of Alzheimer’s disease (AD) treatment. On January 22, 2026, an independent study published online in the prestigious international journal Cell revealed the novel potential of Neu-001 in treating Alzheimer’s disease.
Alzheimer’s disease (AD) is the most common neurodegenerative disorder globally, affecting tens of millions of patients and their families. Despite significant progress in understanding disease mechanisms in recent years, there remains a critical scarcity of effective therapeutic drugs.
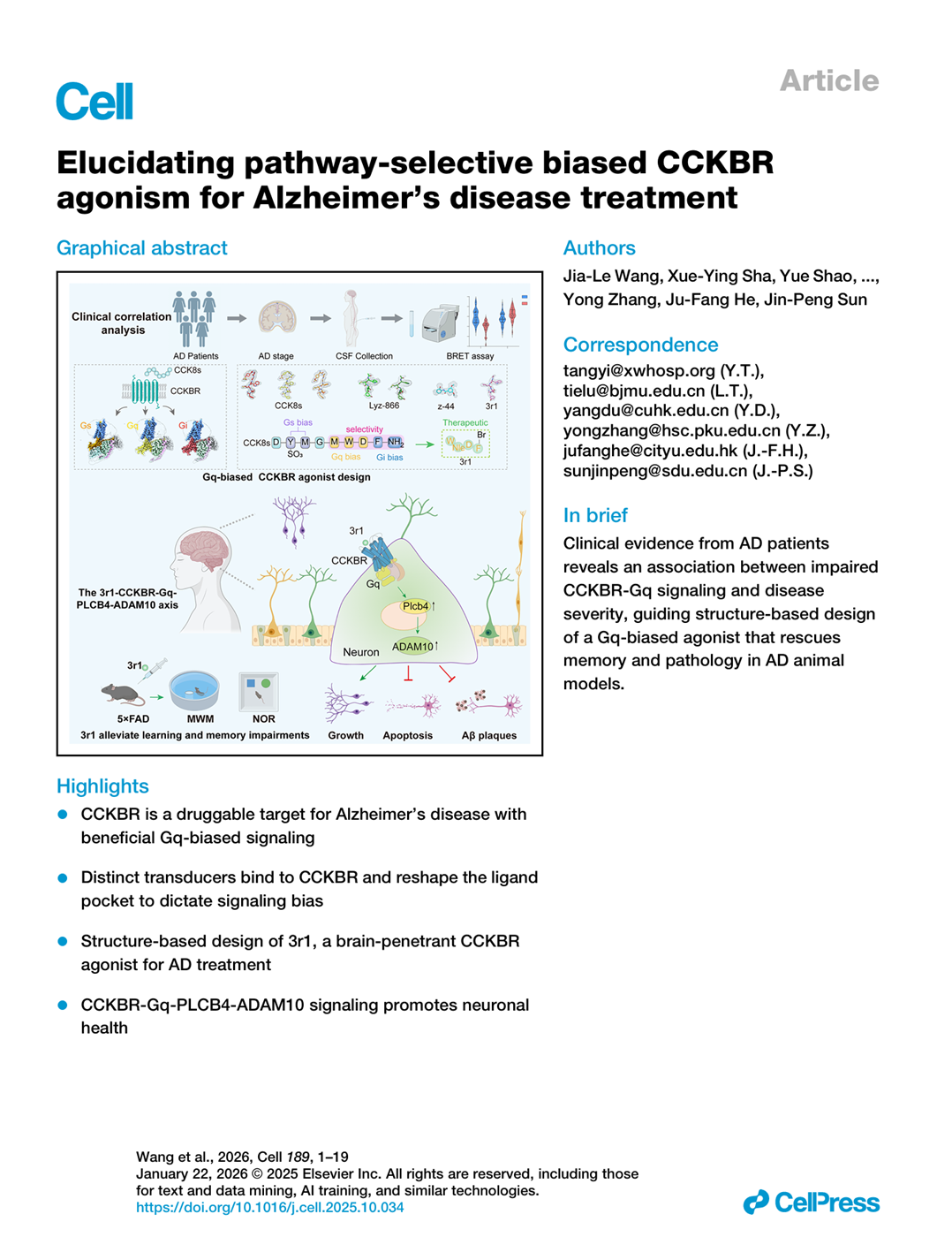
On January 22, 2026, Cell published a research paper titled “Elucidating pathway-selective biased CCKBR agonism for Alzheimer’s disease treatment.” The study was jointly completed by multiple teams, including Professor Sun Jinpeng of Shandong University, Professor He Jufang of City University of Hong Kong, and Professor Zhang Yong of Peking University. The research highlights the new therapeutic potential of the drug molecule Neu-001 (referred to as “3r1” in the paper), which was developed by the Centre for Regenerative Medicine and Health (CRMH), Hong Kong Institute of Science and Innovation, Chinese Academy of Sciences.
By analyzing cerebrospinal fluid (CSF) samples from 78 AD patients, the research team discovered that the Gq signaling pathway activity of the cholecystokinin B receptor (CCKBR) is negatively correlated with disease severity. Based on this clinical finding, researchers utilized cryo-electron microscopy (Cryo-EM) to resolve the fine structures of CCKBR in complex with different G-protein subtypes, elucidating the molecular basis for pathway-selective activation. In 5×FAD Alzheimer’s disease model mice, Neu-001 significantly improved learning and memory capabilities, reduced brain amyloid plaque deposition, and lowered Tau protein phosphorylation levels by selectively activating the CCKBR-Gq-PLCβ4-ADAM10 signaling pathway.
Neu-001 was developed by Professor Micky Tortorella’s research group under Research Project 3 (RP3) at the Center. It was originally intended for the treatment of amblyopia in adults and children. For the amblyopia indication, the drug received Investigational New Drug (IND) approval from both China’s National Medical Products Administration (NMPA) and the U.S. Food and Drug Administration (FDA) in 2025, and has currently completed Phase I clinical trials.
The publication of this Cell paper not only independently validates the molecular mechanism of action of Neu-001 but also unveils its novel application prospects in the field of neurodegenerative diseases. NeuDirection Limited will continue to advance the clinical development of Neu-001 for the amblyopia indication while simultaneously evaluating its development potential for Alzheimer’s disease and other neurodegenerative disorders. This demonstrates the immense promise of the “drug repurposing” translational medicine strategy in the field of neuroscience.