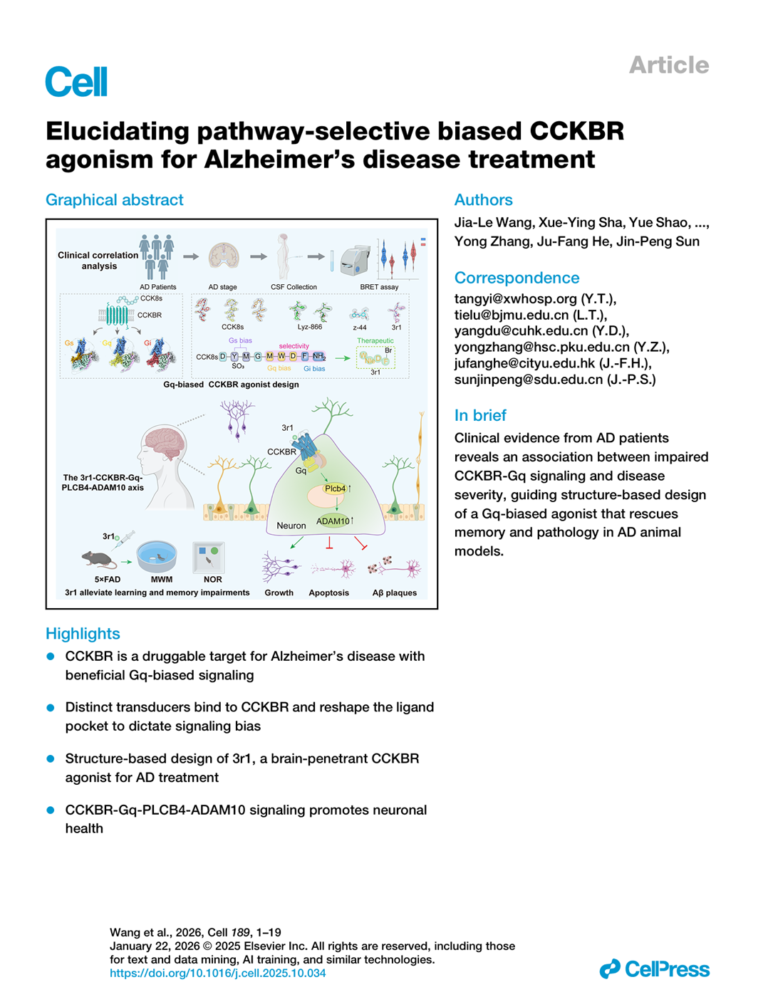
Groundbreaking Discovery in Cell: NeuDirection Limited’s Lead Asset Neu-001 Demonstrates New Potential for Alzheimer’s Disease Treatment
NeuDirection Limited has achieved a significant scientific breakthrough with its lead asset, Neu-001, in the field of Alzheimer’s disease (AD) treatment. On January 22, 2026, an independent study published online in the prestigious international journal Cell revealed the novel potential of Neu-001 in treating Alzheimer’s disease. Alzheimer’s disease (AD) is the most common neurodegenerative disorder globally, affecting tens of millions of patients and their families. Despite significant progress in understanding disease mechanisms in recent years, there remains a critical scarcity of effective therapeutic drugs. On January 22, 2026, Cell published a research paper titled “Elucidating pathway-selective biased CCKBR agonism for…



